

Gene therapy in SCD

Gene therapy overview
Gene therapy offers hope for people living with genetic diseases like sickle cell disease (SCD).
Gene therapies are interventional therapies that use genetic material to treat a disease.1,2
Within the broad class of treatments that are considered gene therapies, there are several approaches at various stages of research and development. The 2 approaches for SCD currently in advanced phases of research are gene addition and gene editing.1,3 Gene therapies for SCD are investigational and not FDA-approved. Safety and efficacy have not been established.

Gene addition
Gene addition is a specific gene therapy approach being studied where genetic material is added to a person’s hematopoietic stem cells to alter production of a functional protein. This research approach is common for monogenic diseases, such as SCD, because these diseases are caused by a single gene mutation.1,2,4
The process of adding modified copies of a gene into a person’s stem cells is accomplished by using a viral vector. Once this vector has delivered functional copies of a modified gene into the nucleus of the targeted cell, the cell is able to produce functional proteins.1

WHY USE A VIRAL VECTOR?
Viral vectors are the most common vectors used in both FDA-approved gene therapies and investigational gene therapies. Gene therapy utilizing lentiviral vectors (LVVs) in SCD is currently investigational. Since the early 1980s, research has investigated the ability of LVVs to infect host cells and transduce genetic information.5,6
The most researched type of LVV is based on the human immunodeficiency virus (HIV), which is naturally designed to efficiently deliver genetic material into cells. The HIV virus has been extensively studied, and its genome has been sequenced so that a blueprint has been developed for each coding region and its subsequent function. Only the sections of the HIV viral blueprint that enable packaging and delivery of genetic material are used for the creation of LVVs. The parts of HIV that code for viral infection and replication are not used, preventing LVVs from causing a viral infection. As research has continued in LVVs over the past few decades, ongoing refinements have been made to improve the safety and durability of these vectors as delivery systems for gene therapies.1,6,7
GENE ADDITION MECHANISM OF ACTION
In the gene addition techniques that are being researched for SCD, stem cells collected from a patient are transduced with a viral vector containing a modified hemoglobin gene. The introduction of this gene allows for the production of functional hemoglobin in the patient’s own red blood cells.1,6,7


Gene editing
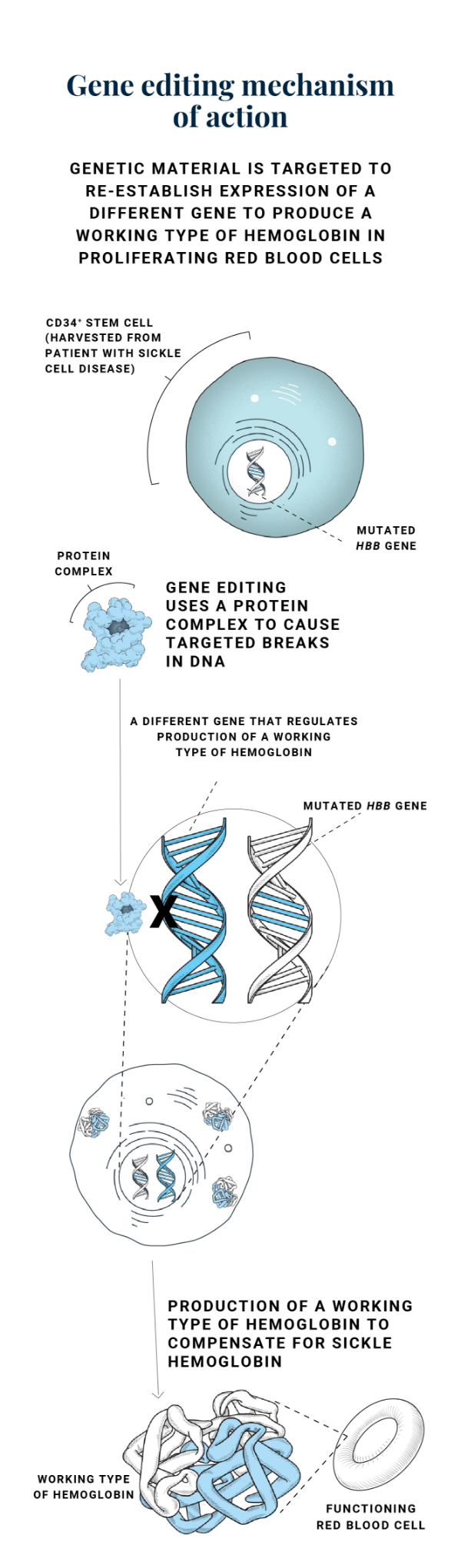
Another clinical approach being studied in gene therapy in SCD is gene editing. Unlike gene addition, where copies of a modified gene are added, gene editing can target a specific gene in a person with a monogenic disease. Gene editing works by creating targeted breaks in the deoxyribonucleic acid (DNA) sequence of a gene in order to correct or modify its function. Some gene editing techniques contain instructions to repair the break and some do not, but in either case, the goal is for the repaired gene to have a therapeutic effect.8,9
The gene editing research furthest along in clinical trials utilizes the technology known as CRISPR-Cas9, an acronym for the Clustered Regularly Interspaced Short Palindromic Repeats system.3,10

GENE EDITING MECHANISM OF ACTION
As an example of gene editing, CRISPR-Cas9 works with a 2-part system that encompasses a guide ribonucleic acid (RNA) and an enzyme. In the initial step, the RNA serves as a guide to hone into a specific DNA sequence while the enzyme subsequently creates the DNA breaks. The cell has inherent repair mechanisms to fix these breaks so that specific insertions or deletions can occur.11
In the case of SCD, gene editing does not work directly on the mutated HBB gene. Instead, it addresses a different gene that regulates the production of a working type of hemoglobin to compensate for the levels of HbS being made.12

Other types of gene therapy
Aside from gene addition and gene editing, there are other gene therapy techniques still in the early phases of research and clinical development.
BASE EDITING
A gene therapy technique that takes the 4 bases contained in our DNA (adenine, cytosine, guanine, and thymine) and has the potential to make precise single base pair changes without the need to create a break in the genetic sequence.13
GENE SILENCING
Rather than breaking or editing a DNA sequence, gene silencing (also known as gene inactivation) is a technique where the targeted gene is either turned off or down through a post-transcriptional RNA interference mechanism.14
Potential risks of gene therapy
While the safety of gene therapy is continuously being evaluated, there are adverse events that may be associated with treatment. Different types of gene therapies may carry different risks, and research continues to provide more clarity into these risks.
Some potential risks of gene therapy include:
INSERTIONAL ONCOGENESIS
In gene addition, the new, modified gene from the viral vector inserts into the DNA of a patient’s stem cell among their other genes. This process is called integration. Most integration causes no harm to the cells or the patient. However, there is a chance that the integration of the new gene may change the activity of nearby genes. If one of these genes controls cell growth, the integration of the new gene may cause uncontrolled cell proliferation after it is returned to the patient, possibly resulting in cancer. Insertional oncogenesis is the term used for a cancer that has been caused by the new DNA inserting in an unwanted location.15,16
UNINTENTIONAL GENE INACTIVATION OR OFF-TARGETING EFFECTS
With gene editing, it may be possible that unsolicited DNA breaks occur in the gene of interest, or that the breaks occur in another gene that is not the original therapeutic target. Either situation could potentially result in unwanted effects, such as disabling a tumor-suppressing gene or activating an oncogenic one. Other types of gene therapies, including gene addition, base editing, and gene silencing, can also carry the risk of affecting other genes that are not the original intended target of treatment.15
Gene therapy treatment process
There are a number of key steps to gene therapy treatment as a class approach.


PART 1:
CONSULTATION
Gene therapy in SCD is investigational and not FDA-approved. The only way to receive treatment with gene therapy currently is to participate in a clinical trial, where patients must meet strict eligibility criteria and be informed about the potential risks and benefits of being part of the trial. If your patient is considering enrolling into a gene therapy clinical trial, you may start conversations depending on their medical history and impact of their disease, life goals, and eligibility.
The consultation process may involve several discussions over a significant period of time. These discussions can include multiple consultations with specialists/physicians at a specialized treatment center with expertise on gene therapy. Patients will need to consider the risks and potential benefits of therapy, as well as the impact of the treatment process on their daily life, before reaching a decision.
DISCUSSING WITH YOUR PATIENT
- Are you aware of the risks and potential benefits of gene therapy?
- What are your life/professional goals that could help us consider if gene therapy is an appropriate treatment for you?
- All transplant-based treatments require a chemotherapy regimen, which will impact fertility, to clear out the existing bone marrow so that new stem cells can engraft properly—do you have any questions or concerns about this part of the treatment process?
- The entire process of gene therapy may be over the course of months—what are the considerations that we need to consider for your work, your family, the time that is required for treatment, and your healthcare coverage?


PART 2:
PREPARATION
If a decision is made to pursue gene therapy, patients will need to undergo informed consent before starting the treatment process. Patients may need to undergo mobilization and apheresis at a specialized treatment center for stem cell collection. These cells will then be sent to a lab where the therapy will be manufactured.
DISCUSSING WITH YOUR PATIENT
- Multiple rounds of collection may be required in order to acquire enough cells for gene therapy manufacturing—do you have any questions regarding how these cells will be used in the gene therapy process?


PART 3:
TREATMENT
Once the therapy has been manufactured, the patient will be brought back into the specialized treatment center for treatment. As with all transplant-based treatments, chemotherapy will be required prior to the gene therapy treatment.
DISCUSSING WITH YOUR PATIENT
- You’ll be at the hospital for some time to receive treatment and to recover—have you considered the time that is required for treatment and who you want to be with you during this time?
- Do you have any additional concerns about conditioning with chemotherapy?


PART 4:
RECOVERY/FOLLOW-UP
Once treatment is complete, the patient will need to remain in the hospital for several weeks and be monitored for signs of a successful engraftment—a critical stage of the treatment. After engraftment has occurred, recovery will differ from patient to patient, but it’s important to make sure they understand the process and what they can expect after they leave the hospital. Follow-up can last for a number of years. Patients may be asked to enroll in a long-term registry to assess efficacy and safety of gene therapy over time.
DISCUSSING WITH YOUR PATIENT
- What questions or concerns do you have about the recovery process?
- What should be considered in your follow-up in terms of frequency of check-ups, and preferred communication methods?
- What are some ways you can help keep track of how treatment has affected you in terms of your physical, emotional, and mental health?
As gene therapy research continues to evolve, the
future of SCD care may evolve as well
As gene therapy research continues to evolve, the future of SCD care may evolve as well
References
1. National Institutes of Health. Genetics Home Reference. Help me understand genetics. Accessed March 21, 2022. https://medlineplus.gov/download/genetics/understanding/primer.pdf 2. FDA Commissioner. What is gene therapy? How does it work? US Food and Drug Administration. Accessed March 31, 2022. https://www.fda.gov/consumers/consumer-updates/what-gene-therapy-how-does-it-work 3. Kanter J, Falcon C. Gene therapy for sickle cell disease: where we are now? Hematology Am Soc Hematol Educ Program. 2021;2021(1):174-180. 4. Biology Online Dictionary. Monogenic disease. Accessed March 31, 2022. https://www.biologyonline.com/dictionary/monogenic-disease 5. STAT Reports. The STAT guide to viral vectors, the linchpin of gene therapy. STAT News; 2019 6. Durand S, Cimarelli A. The inside out of lentiviral vectors. Viruses. 2011;3(2):132-159. 7. Warnock J, Daigre C, Al-Rubeai M. Introduction to viral vectors. In: Merten O-W, Al-Rubeai M, eds. Viral Vectors for Gene Therapy: Methods and Protocols, Methods in Molecular Biology. Springer Science+Business Media; 2011. 8. Collins M, Thrasher A. Gene therapy: progress and predictions. Proc Biol Sci. 2015;282(1821):770-788. 9. Guha TK, Wai A, Hausner G. Programmable genome editing tools and their regulation for efficient genome engineering. Comput Struct Biotechnol J. 2017;15:146-160. 10. Encyclopedia Britannica. Gene editing. Accessed March 31, 2022. https://www.britannica.com/science/gene-editing 11. National Cancer Institute. NCI dictionary of cancer terms: CRISPR-Cas9. Accessed March 31, 2022. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/crispr-cas9 12. Demirci S, Leonard A, Essawi K, Tisdale JF. CRISPR-Cas9 to induce fetal hemoglobin for the treatment of sickle cell disease. Mol Ther Methods Clin Dev. 2021;23:276-285. 13. Rees H, Liu D. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat Rev Genet. 2018;19(12):770-788. 14. Taber’s Online Medical Dictionary. Accessed March 31, 2022. http://www.tabers.com/tabersonline 15. Goswami R, Subramanian G, Silayeva L, et al. Gene therapy leaves a vicious cycle. Front Oncol. 2019;9:297 16. American Society of Gene & Cell Therapy. Sickle Cell Disease. Accessed May 26, 2022. https://patienteducation.asgct.org/disease-treatments/sickle-cell-disease

